Workshop #PR121 “Detection of antibodies to COVID-19 by ELISA”
Workshop #PR121 “Detection of antibodies to COVID-19 by ELISA”
Institute of Biotechnology National Academy of Sciences of the Kyrgyz Republic
Concluded on September 20-24, 2021
 |
Serological diagnostics for coronavirus infection is developing rapidly, but has not yet become widespread in Kyrgyzstan. To strengthen laboratory services, the Ministry of Health of the Kyrgyz Republic were selected qualified laboratory specialists from the Naryn, Issyk-Kul and Chui regions to the Institute of Biotechnology of the National Academy of Sciences of the Kyrgyz Republic.
The training course consisted of two parts - lecture and practical (25 academic hours). Both parts of the training were conducted at the Institute of Biotechnology of the National Academy of Sciences of the Kyrgyz Republic (IB NAS KR). The conference hall was used for lectures and the laboratory of the institute for practical work.
Table 1.
|
No |
Full name |
Place of work |
Position |
|
1 |
Aalieva Tilegul |
Naryn Regional United Hospital, Diagnostic laboratory |
Laboratory assistant |
|
2 |
Ybyshova Kulnara |
Naryn Regional United Hospital, Diagnostic laboratory |
Laboratory doctor |
|
3 |
Kasmalieva Atyrkul |
Naryn Regional United Hospital, Diagnostic laboratory |
Laboratory assistant |
|
4 |
Zhumalieva Lenakan |
Chui Regional Center HIV |
Laboratory assistant |
|
5 |
Kalkesheva Gulyar |
ZhaiylCenter for General Practice |
Head of laboratory |
|
6 |
Kaseinova Makhabat |
Balykchy Center for General Practice |
Laboratory doctor |
|
7 |
Imanalieva Ziyagul |
Aksuu Center for General Practice |
Laboratory doctor |
|
8 |
Tyulegabylova Mairamkul |
Issyk-Kul Regional United Hospital, Central laboratory |
Laboratory doctor |
|
9 |
Baychubakova Nazira |
Institute of Biotechnology NAS KR |
Researcher |
|
10 |
Konurbaeva Rakhat |
Institute of Biotechnology NAS KR |
Researcher |
Trainers:
· Anara Umralina – Doctor of biological sciences
· Tatiana Chernysheva – Candidate of biological sciences
· Nurulanbek Sultanaliev – Candidate of veterinary sciences
· Sergey Hegay – PhD
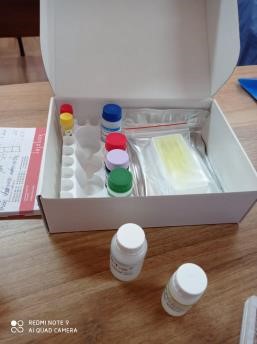
Materials and methods. As the test material were used 3 native samples with known concentration for IgM - positive (# 807578, CP = 11.6), IgG positive (# 806894, CP = 7.2) and 1 - negative (# 11122).
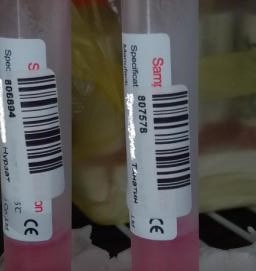
Positive samples for SARS-CoV-2 IgM, IgG
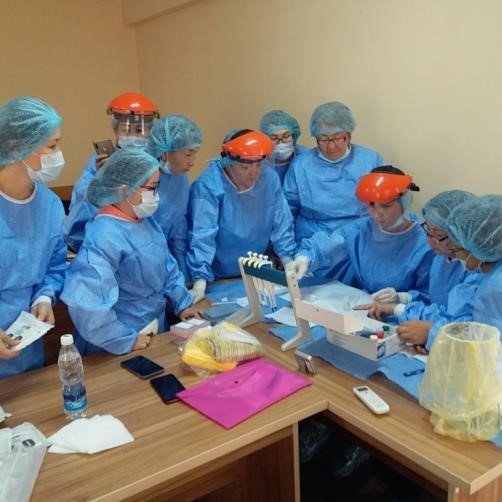
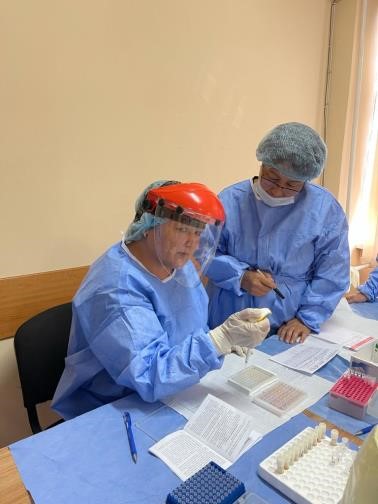
In the training, in addition to the control samples, serum obtained from each of the participants was used. It was noted that the participants in the training know how to select biomaterials. Blood vacutainers, alcohol wipes and other materials were used to collect blood samples.
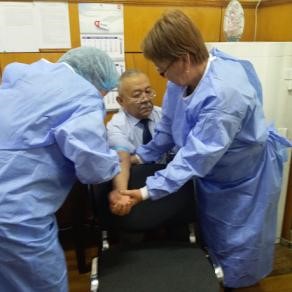
Taking blood from the director of the institute by the participants of the training

Opening of the seminar, director of the institute
prof. Asankadyr Zhunushov
The lecture material was prepared by the trainers and provided to the course participants in the form of visual presentations. Discussions were held at the end of each presentation.
|
|
|
|
|
|
Laboratory work.
Laboratory studies were carried out in the laboratories of the Institute of Biotechnology of the National Academy of Sciences of the Kyrgyz Republic. There are rooms for receiving and registering samples, sample preparation, obtaining distilled water, conducting incubation, automatic washing (washer) and recording results (Reader spectrophotometer), disposal rooms (washing, autoclave) and storage of biosamples (-20C, -80C), etc.
The procedure for setting up an analysis for the determination of antibodies for COVID-19 was carried out according to the instructions of the manufacturer of the test kit. For the training, we used ELISA SARS-CoV-2 for detection antibodies IgG and ELISA SARS-CoV-2 for detection antibodies IgM from Vektor Best, Russia. When performing laboratory work, safety rules were observed when working with infectious material.
|
|
|
|
Discussion of the activities of the laboratory of cultures of cells and tissues |
Discussion of the work of the laboratory of microbiology |
|
|
|
|
Discussion of work in the laboratory of molecular biology and serology |
|
|
|
|
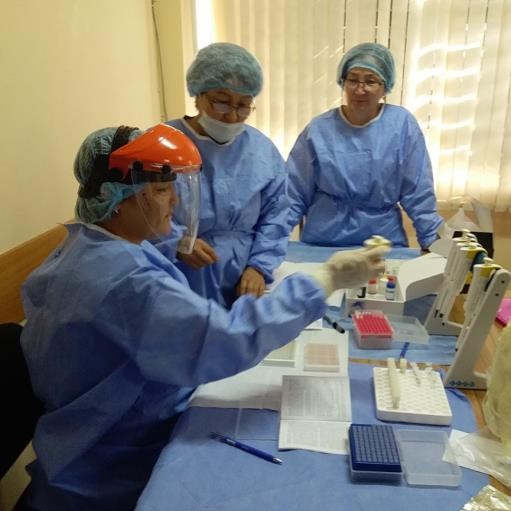
Setting up an ELISA test to detect antibodies to SARS-CoV-2
|
|
|
|
Test-system ELISA SARS-CoV-2 for detection antibodies IgG |
Test-system ELISA SARS-CoV-2 for detection antibodies IgM |
|
|
|
|
Filling in research protocols |
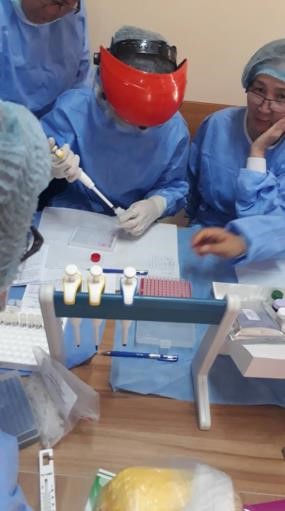
Preliminary dilution of samples with substrate |
Participants were given research protocol blanks before starting the test. The participants were divided into two groups of 5 people. Each group completed the protocol for the study ELISA SARS-CoV-2 for detection antibodies IgM and ELISA SARS-CoV-2 for detection antibodies IgG.
|
|
 |
|
Test ELISA setup |
Adding solution to the wells for preliminary dilution of control samples |
|
|
|
|
Prepare samples for placement on a production plate |
Inserting samples from a pre-diluted plate into a production plate |
Each participant contributed their own serum, positive sample, and negative sample.
|
|
|
|
|
|
Results.
The results were recorded using a Sunrise reader at a wavelength of 450-620 nM.
Protocol of the results obtained for the detection of SARS-CoV-2 IgG antibodies
Protocol of the results obtained for the detection of SARS-CoV-2 IgM antibodies
Each group of participants performed calculations on the validity of the test. The coefficient of positivity of the samples and control samples was calculated.

|
|
|
|
|
Work in groups to take into account the results obtained |
||
Positive results were obtained for the detection of SARS-CoV-2 IgG antibodies.
The internal quality control assessment was carried out using a computer program. For an illustrative example, a Shewhart control chart was built with the obtained data. The OD of the positive control sample (No. 807578, CP = 11.6 IgM) was used as ICS. The graph shows a normal distribution with an average CP value of 9.8.
At the end of the training, the participants received certificates.
|
|
|
|
Ceremony of awarding certificates |
|
Conclusion.
Participants received hands-on training in the detection of antibodies to COVID-19 by ELISA. Practical demonstration methods were shown from sampling, sample preparation, analysis setting, recording and interpretation of results.
Based on the results obtained, it was revealed that all training participants from public health institutions have a high level of IgG antibodies with an average CP = 15. This good result indicates that the body has produced antibodies after being vaccinated against the coronavirus.
For the first time, participants make ELISA test to detect antibodies to coronavirus and indicated the need for such practical trainings for laboratory specialists.
Acknowledgement.
Participants in the training, including trainers and trainees, express their deep gratitude to:
• Mr. Park Sungbeen, National Research Foundation of Korea (NRF) in providing confidence in delivering training and funding;
• ISTC specialists in Kazakhstan and Kyrgyzstan in providing technical assistance in the successful conduct of the training.